B Cells
B cells are the antibody-producing cells in our immune system. B cells can release cytokines and present antigens to T cells thereby regulating the immune response.
B cells develop in the bone marrow from hematopoietic stem cells (HSC) before egressing out into the periphery and further maturing in secondary lymphoid organs (spleen, lymph nodes). Upon antigen encounter, B cells develop either into short-lived plasma cells via the extrafollicular pathway or develop through the germinal center into long-lived plasma cells or memory B cells.
Defects in B cell function can result in autoimmune diseases, immunodeficiency and immune dysregulation.
Our Laboratory
Our laboratory is interested in human B cell development, maturation and activation in physiological and pathological conditions.
We study patients with inborn errors of immunity with mutations in genes affecting B cells, and with autoimmunity. In particular we study ANCA associated vasculitis, rheumatoid arthritis, anti-phospholipis syndrome.
We have developed a unique expertise in in vitro modelling of early and late human B cell development that we combine with molecular and biochemical tools: spectral flow cytometry, scRNA seq, CRISPR Cas9 gene manipulation, single cell immortalization.
Research Focus
- Regulation of early B cell development: signaling and trascription factors driving B lymphopoiesis
- B cell fate decisions in the periphery: role of TNF-receptor superfamily members
- Interaction of human B cells with the extracellular matrix: how locations affects B cell maturation
- Mechanisms of disease in monogenic defects leading to autoimmunity
- B cell function in rheumatological diseases
- Specific impact of novel targeted therapies on human B lymphocytes
See list of projects on early and late/peripheral B cell development below.
Projects on early human B cell development
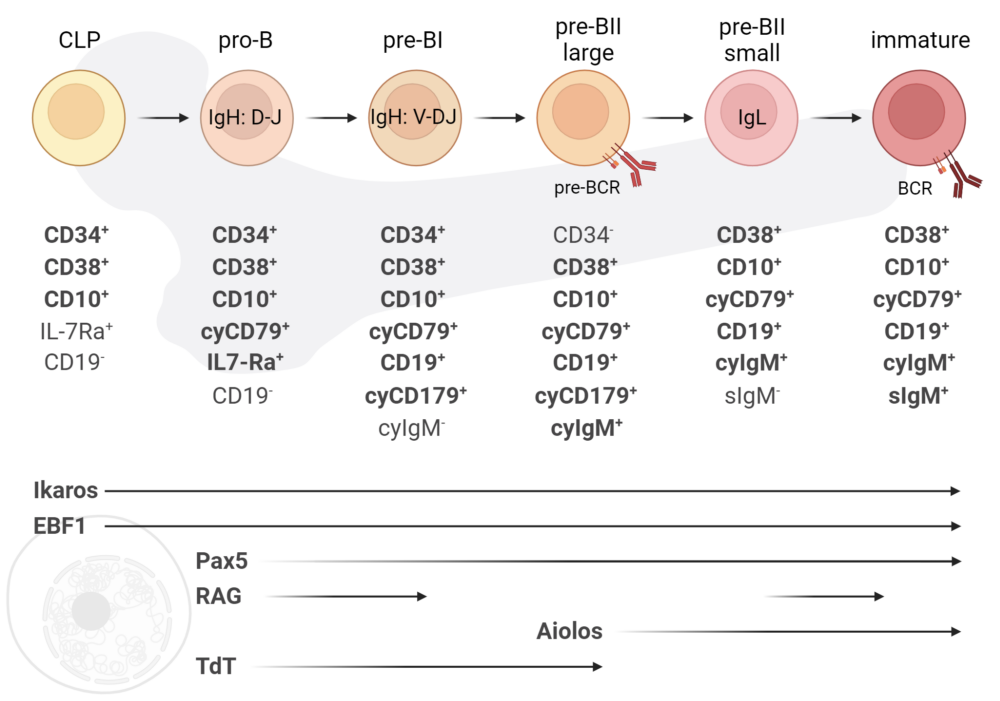
Ikaros/Aiolos
We elucidate the role of Ikaros and Aiolos transcription factors during human early B cell development by increasing their proteasomal degradation via a molecular glue, named iberdomide (lenalidomide analogue). Since our in vitro platform recapitulates the complete human early B cell development, we hereby are able to identify potential effects of iberdomide treatment in patients.
JAK/STAT signaling
The usage of JAK inhibitors (JAKinibs) in patients uncovered a transient increase of peripheral B cells. Here, we decipher the importance of JAK/STAT signaling during human early B cell development by using approved JAKinibs (tofacitinib, ruxolitinib) in our in vitro system.
IL-7 signaling
in collaboration with Mirjam van der Burg, Netherlands
The IL-7 signaling was shown essential for murine B lymphopoiesis, however its role in humans is not yet fully resolved. Therefore, we are interested in further unveiling the importance of IL-7 during human B cell development using the BM of IL-7R deficient patients and our in vitro platform.
Read the paper here.
Projects on peripheral human B cell development
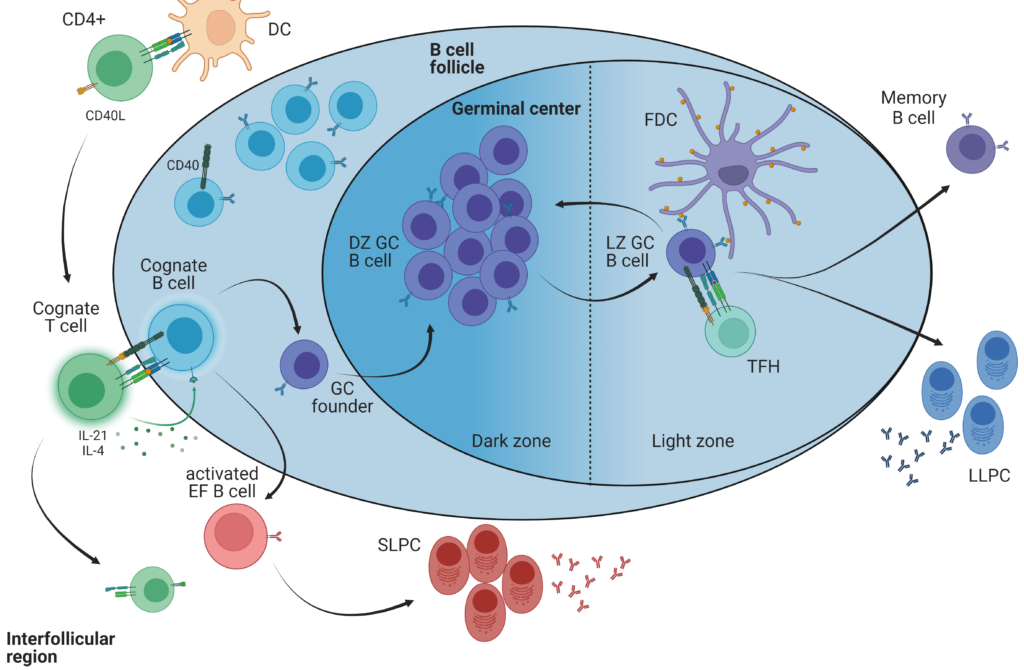
Non-apoptotic FAS signaling
funded by DFG / SFB1160
In this project, we uncover the importance of non-apoptotic FAS signaling in guiding peripheral B cells into the extrafollicular or the germinal center fate by modulating CD40-induced mTOR activity.
ECM-B cell interaction
in collaboration with Alexander Nyström, Freiburg and Dimitra Kiritsi, Thessaloniki
We investigate how the expression of integrins and their interaction with the respective ligands modulate B cell fate decisions in human peripheral B cells.
Intracellular signaling in primary immunodeficiencies (PIDs)
We identify intracellular phosphorylation patterns of major immune signaling pathways to be used in the diagnosis of inborn errors of immunity.
TRAIL(R) signaling
funded by DFG / TRR353
We investigate the outcome of TRAIL-R signaling in human B cell development. We study the signaling and molecular context of survival/cell death decision in response to TRAIL.
